Introduction: Treatment of patients with AML has evolved in the past decade, with development of novel drugs and increased knowledge about the genetic basis of disease. However, results of therapy in AML in LMICs have lagged those seen in more developed countries. A recently published paper highlighted the worse outcome of pts with AML treated in two public healthcare hospitals in Brazil compared with pts treated at an academic center at Oxford, UK, with lower overall survival (OS), lower rates of HSCT and a longer time to HSCT. 1 We hypothesized, however, that better outcomes could be seen in private healthcare institutions in Brazil with a higher rate of HSCT in first remission.
Methods: We reviewed medical records of 122 patients with a diagnosis AML treated at Hospital Israelita Albert Einstein in São Paulo, Brazil, from 2010 until 2023. OS was defined as time from diagnosis until death from any cause and estimated by the Kaplan-Meier method. Risk was evaluated by the ELN 2022 risk classification.
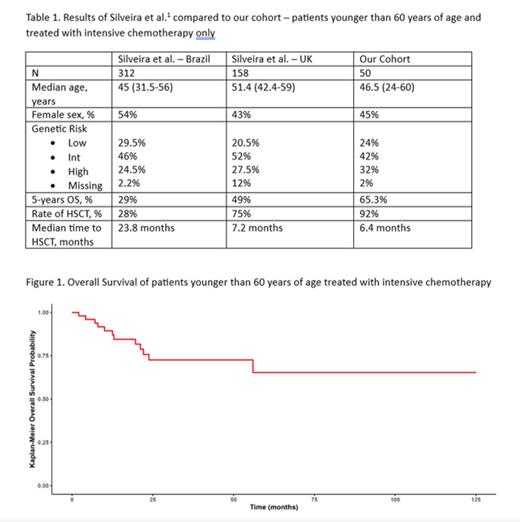
Results: A total of 122 consecutive patients were included. Median age was 63 years (range 24-91 years), and 45% were female. Per the ELN 2022 risk classification, 17% of patients were low risk, 33% were intermediate risk and 50% were considered high risk cases. Intensive chemotherapy was used in 62.5% of cases, while low-intensity regimens were employed in 37.5% of cases, including venetoclax in 21.4% of patients. After a median follow-up of 36 months, median OS of the whole cohort is 25.2 months (95% CI 19.2-83.8 months), and 3-years OS is 44% (95% CI 34-55%). As expected, patients in the high 2022 ELN risk category had lower 3-years OS probability (15.6% vs. 47.3%/91.6% for intermediate- and low-risk, respectively). Eighty-two patients (67.2% of the cohort) underwent HSCT, and 59% were in MRD-negative complete remission (CR) prior to HSCT. We next analyzed outcomes in patients younger than 60 years of age who received intensive chemotherapy, the subset of patients analyzed by Silveira et al. 1 Results are summarized in Table 1 and figure 1. The outcomes of our patients compare favorable to what has been reported here in Brazil and, like the data from the UK center, has a high rate of HSCT with a low time to transplant.
Conclusion: Our results demonstrate that it is feasible to obtain favorable outcomes in patients with AML in a LMIC with a high rate of HSCT in first CR. It is also important to mention that our results also reflect the reality of a private healthcare institution in Brazil. However, even after considering this, our results carry an important message. There is limited availability of clinical trials and novel medications for AML in LMICs compared to the developed world, and options in the salvage setting are usually limited to intensive chemotherapeutic regimes. Allogeneic HSCT is a technique that is readily available in most countries and has the potential to cure AML, particularly when employed in first CR. We believe that physicians and institutions treating patients with AML in a LMIC should primarily focus on strategies that improve access to HSCT in first CR for these patients.
References
1. Silveira DRA et al, Leuk Lymphoma 2021 Jan;62(1):147-157
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal